
Fiabilité et conformité pour les systèmes pharmaceutiques
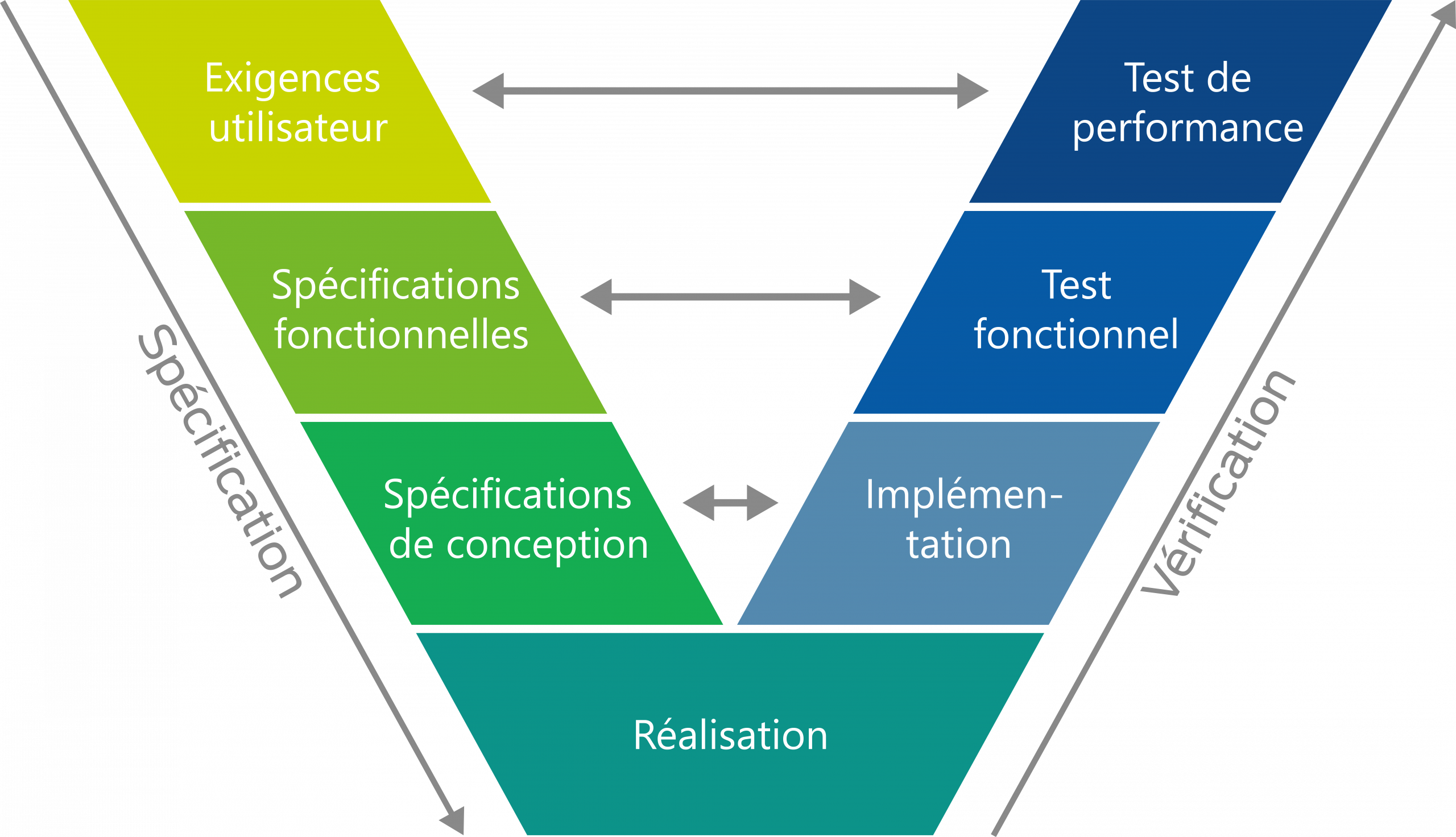
Dans l’industrie pharmaceutique, la documentation complète et la sécurisation des systèmes techniques constituent un élément central des exigences réglementaires. Nos prestations dans le domaine de la qualification garantissent la conformité des installations et des systèmes informatiques aux directives en vigueur, de la conception à la mise en service, en passant par la mise en œuvre.
Nous nous basons sur des normes internationales telles que GMP, GxP et GAMP et créons, grâce à des processus de contrôle structurés et une documentation traçable, les bases d’un fonctionnement sûr, stable et conforme aux audits. Notre approche tient compte à la fois de la fonctionnalité technique et de l’intégrité réglementaire, et aide les entreprises à garantir durablement la qualité et la conformité.
Qualification
- Planification, conception et vérification conformément aux exigences réglementaires (par exemple, GMP, GxP, GAMP)
- Qualification
- Validation
- Qualification de la conception (DQ)
- Qualification de l’installation (IQ)
- Qualification opérationnelle (OQ)
- Validation du système informatique (CSV)
Engineering Support – Prestations d’accompagnement de projet
De la planification à la mise en œuvre, nous accompagnons vos projets grâce à notre savoir-faire technique et à notre connaissance approfondie des processus, de la qualité et de l’efficacité.
Informez-vous et découvrez-en plus sur notre Engineering Support.
Votre projet. Notre engagement.
Contactez-nous.









